by aisla@raikee.fi | Nov 30, 2020 | News
First-in-Human clinical study with birch pollen hypoallergen DM-101 has been restarted after a temporary halt. In early 2020, Desentum initiated a clinical study to evaluate the safety and tolerability of DM-101, a hypoallergen designed for treating birch pollen...

by aisla@raikee.fi | Mar 26, 2020 | News
Clinical study initiated in January 2020 with birch pollen hypoallergen DM-101 was suspended in March. The main reason for suspension is that the corona virus epidemic has brought about restrictions in the operations at the study site. In January, Desentum initiated a...
by aisla@raikee.fi | Jan 31, 2020 | News
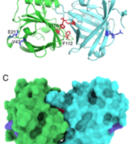
The latest open access paper about our hypoallergen development is available at Nature Scientific Reports. The paper, titled “Development of hypoallergenic variants of the major horse allergen Equ c 1 for immunotherapy by rational structure based...

by aisla@raikee.fi | Nov 1, 2019 | News
Desentum is about to initiate a first-in-human clinical trial with its birch pollen hypoallergen designed to improve immunotherapeutic treatment of birch pollen allergy. In a funding round arranged by Springvest Oy, the company raised 4 million euros that it intends...

by aisla@raikee.fi | Sep 18, 2019 | News
Preparations are currently being finalized for the first clinical study with DM-101 hypoallergen, Desentum’s lead product candidate for the treatment of birch pollen allergy. DM-101 (rBet v 1 dm) is a genetically engineered birch pollen allergen that is designed to...

by aisla@raikee.fi | Oct 29, 2018 | News
Desentum has been awarded a 1.9 M€ grant from the EIC pilot SME Instrument for a project titled AllergyVAX. The two-and-a-half-year project, starting at the end of 2018, includes early clinical trials of Desentum’s lead product candidate, an immunotherapeutic vaccine...







