Technology
Allergies are often managed by avoiding the allergen or by using medication to relieve symptoms. However, avoidance is not always possible, and medication may not offer sufficient symptom control. Allergen immunotherapy (AIT) is the only available treatment that affects the underlying condition by increasing tolerance to the allergen, but the treatment typically takes years, has unpredictable efficacy, and can involve a risk of severe allergic reactions during treatment.
Safe and successful allergen immunotherapy with recombinant hypoallergens
Desentum ’s hypoallergens are designed to induce a protective immunological response without compromising safety during treatment:
- Targeted modifications in the allergen protein to reduce IgE cross-linking, thus reducing allergenic potential during immunotherapy
- Native conformation of the hypoallergen retained to ensure that the protective immunological response induced by the hypoallergen also works against natural allergens
- Novel formulation for controlled release of the active substance after injection, further increasing safety
- Recombinant production for consistent, high-quality manufacturing of hypoallergens
The goal is safe and effective subcutaneous allergen immunotherapy with significantly lower number of injections compared to standard subcutaneous AIT.
How allergy and immunotherapy work
Allergies are caused by an overreaction of our immune system. A normally harmless substance – like pollen, food, or cat saliva – will cause the immune system to defend the body against it.
How allergy and immunotherapy work
Allergies are caused by an overreaction of our immune system. A normally harmless substance – like pollen, food, or cat saliva – will cause the immune system to defend the body against it. Type 1 hypersensitivity, mediated by immunoglobulin E antibody (IgE), causes allergic reactions such as sneezing, itchy eyes, running nose, rash, abdominal pain, or vomiting. The most severe allergic reaction, anaphylaxis, can rapidly develop into a life-threatening condition.
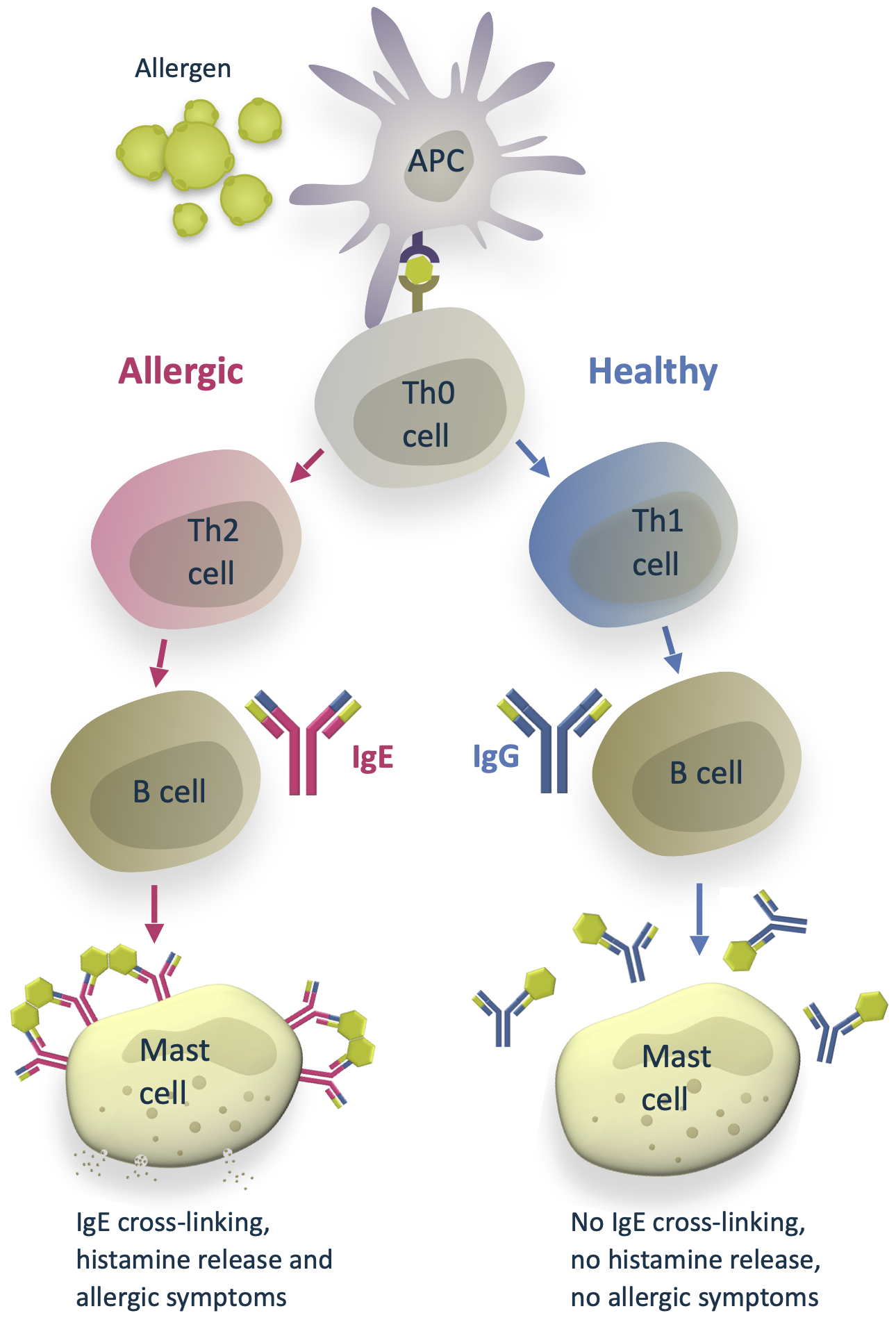
Difference between healthy response and allergy
When the immune system encounters an allergen, antigen-presenting cells (APC) introduce the allergen to naïve T helper cells (Th0).
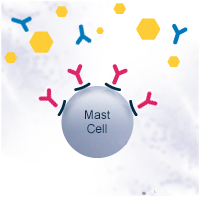
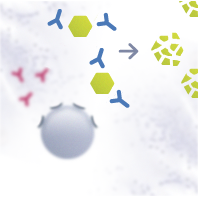
In a healthy individual, the Th0 cells differentiate into T helper type 1 cells (Th1). Th1 cells activate B cells that differentiate into plasma cells and produce allergen-specific immunoglobulin G antibodies (IgG). The IgG antibodies have a protective function. They help to immobilize and destroy the allergens without causing an allergic response.
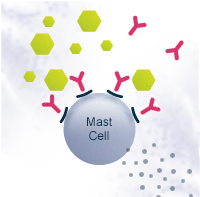
In some people with atopic predisposition, natural allergen exposure can lead to sensitization and allergy. Instead of Th1, the naïve Th0 cells differentiate into T helper type 2 cells (Th2). This path leads to B cells differentiating into plasma cells that produce allergen-specific immunoglobulin E antibodies (IgE). IgE antibodies bind to the surface of mast cells. On subsequent allergen exposure, the allergen cross-links them, and the mast cells release histamine and other cytokines that cause the allergic symptoms.
Allergen immunotherapy (AIT)
The goal of allergen immunotherapy is to re-educate the immune system, thus directing it towards the healthy Th1 response. For many allergies (pollen, house dust mite, animals, bee venom, etc.), AIT has been available for a long time. Traditional allergen immunotherapy is a method where gradually increasing doses of the natural allergen are administered by subcutaneous injections. Alternatively, daily sublingual drops or tablets can be used for some allergies. This desensitizes the body and tweaks the immune response away from the allergic mechanism, towards a healthy response. The treatment is usually continued for 3–5 years, depending on the allergen and the desired outcome.
Immunotherapy increases tolerance to the specific allergen and reduces the need for medication. The effect is sustained even after the treatment period is over. However, traditional allergen immunotherapy with natural allergens takes a long time and the up-dosing requires careful medical supervision to minimize the risk of a serious allergic reaction (anaphylaxis).
For food allergies, availability of immunotherapy is very limited, and the treatment effect is not sustained if the treatment is stopped. The standard of care for most food allergies is allergen avoidance and carrying an epinephrine autoinjector in case of accidental exposure.




The technologies we use allow us to precisely characterize hypoallergen structure and interaction, design targeted modifications to them and evaluate potential product candidates.
Mass spectrometry
Protein crystallography
HRA histamine release assay
Scientific articles
Haka J et al. 2019. Development of hypoallergenic variants of the major horse allergen Equ c 1 for immunotherapy by rational structure based engineering. Sci. Rep. 9(1):20148
Niemi MH et al. 2015. Dimerization of lipocalin allergens. Sci. Rep. 5, 13841; doi: 10.1038/srep13841
Rouvinen J et al. 2010. Transient dimers of allergens. PLoS One. 5(2):e9037
Niemi M et al. 2008. Characterization and crystallization of a recombinant IgE Fab fragment in complex with the bovine beta-lactoglobulin allergen. Acta Crystallogr Sect F Struct Biol Cryst Commun. 64(Pt 1):25-28.
Niemi M et al. 2007. Molecular interactions between a recombinant IgE antibody and the beta-lactoglobulin allergen. Structure 15(11):1413-21.
Product pipeline
We have completed clinical Phase 1 with the lead product candidate, birch pollen hypoallergen DM-101PX. Further clinical development of DM-101PX is ongoing. Each allergy requires its own hypoallergen, so other hypoallergens are in development. The product line will be expanded as we manufacture new modified hypoallergens.
Birch pollen
- Clinical Phase 1 completed
- Novel formulation developed
- Phase 2 planned in 2025
Phase 1
Peanut
- Modified Ara h 2 hypoallergen candidates produced
- Preclinical evaluation ongoing
- Clinical phase targeted in 2026
Preclinical
Grass pollen
- Modified Phl p 1 hypoallergens produced and evaluated
- Drug candidate selection ongoing
Preclinical
Clinical trials
DM-101-C-001
The randomised, double-blinded, placebo-controlled, dose escalation study was designed to evaluate the safety and tolerability of subcutaneous immunotherapy with DM-101 in birch pollen allergic adults. Immunological markers such as allergen-specific IgE and IgG were also analysed.
DM-101 was found to be safe and well tolerated in a dose regimen of 5 ascending doses, and immunological marker results indicated a favourable immunological response.
DM-101-C-002
The randomised, double-blind, placebo-controlled study evaluated three dose escalation regimens, each consisting of ten subcutaneous injections over 10 weeks with DM-101PX or placebo.
The short-course treatment was found to be safe and well tolerated in birch pollen allergic patients and to induce a very strong and sustained allergen-specific IgG4 response. DM-101PX-induced immunoglobulins were found to efficiently block IgE-mediated basophil activation.
DM-101PX is a formulation of DM-101 optimised to support controlled release of the active substance after subcutaneous injection.
